
Nonpolar lipids(triglycerides) are utilized as fuel and to store energy. These categories are divided into non-polar and polar lipids. Ester group is enabling for hydrolysis in the presence of acid, base, including waxes, triglycerides, phospholipids and sphingolipids etc A saponifiable lipid consists of one or more ester groups. A non saponifiable lipid does not break down into smaller molecules through hydrolysis. Lipids categories into two classes: Non Saponifiable lipid and Saponifiable lipids. These non-polar solvents are usually hydrocarbons that dissolve other organic hydrophobic substances. Most lipids are soluble in non-polar solvents. When amphipathic lipids come in contact with whatever their hydrophilic parts stay in contact with water and the hydrophobic parts stay away from water. Such lipids are usually those which make cellular membranes because of the way they behave in water. Therefore, such lipid molecules are called amphipathic. But this is usually a small part of the molecule, the rest of the molecule is usually hydrophobic. Some lipids show a small tendency of being hydrophilic. However the majority of lipids are hydrophobic, that is, they do not interact with water. Most biological substances can interact with water. The study of the function and structure of lipids constitutes a sizable part of the study of organic compounds because they make up such substances as oils, fats, wax, membranes and energy-storing molecules in plants and animals. Put in simple terms, most substances made of lipids will not be miscible with water. A deep understanding of chemistry is essential to anyone interested in modern biological sciences or medicine, so I really encourage you to take the time to work though all of the chemistry material.Lipids are classified as those organic compounds which do not interact with water. 
That may look like a lot of work, but you've probably watched many of the videos already under "Chemistry of life". If you are not familiar with reduction and oxidation states, then I encourage you to start working through the Chemistry material on KhanAcademy: How many moles of carbon are present in a gram of glucose (a "typical" carbohydrate)?ĭo the answers to those two sets of questions help you answer your question? How many moles of carbon are present in a gram of tetradecane - a 14 carbon alkane (a reasonable comparison for the tails of the fatty acids found in food)?
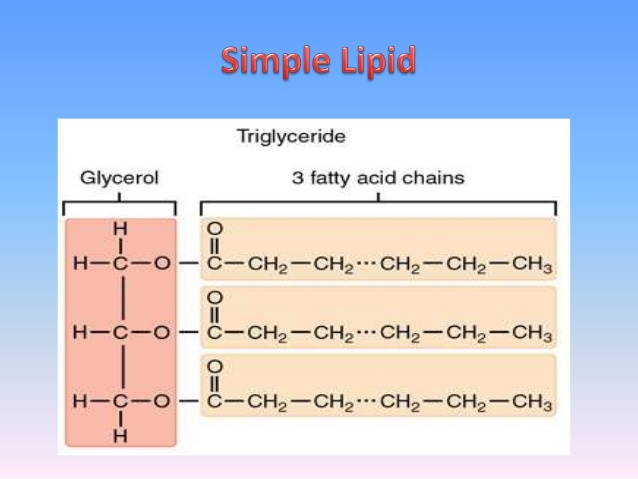
How would you expect this to affect a oxidative process like cellular respiration? How does this compare with the oxidation state of the carbon in carbohydrates (the other group of macromolecules that are often used to store energy)? What is the oxidation state of the carbons in the fatty acid tail? Therefore, I'm going to ask you some questions in response to help you figure out (some of) the answers yourself.

This is a good question, but one that I think you have enough information to answer on your own.







 0 kommentar(er)
0 kommentar(er)
